List Of News
2024-06-24 (CHOQUET Team) : PhD Project Overview: AMPA Receptor Dynamics in Synaptic Plasticity
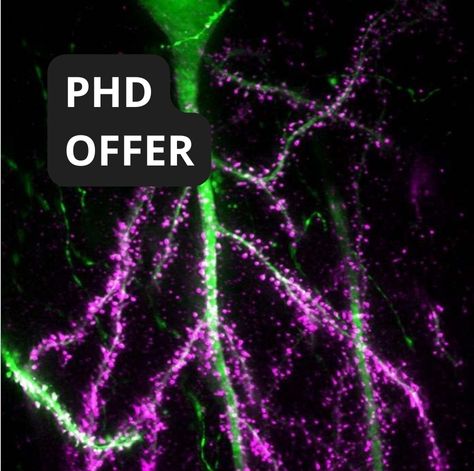
Project Title: Unraveling AMPA Receptor Dynamics in Synaptic Plasticity Using Cutting-edge Imaging and Electrophysiology
Introduction
Synapses, the communication hubs of the brain, rely on their molecular composition and organization for effective information processing. AMPA receptors (AMPARs) are crucial in this process as they mediate fast excitatory synaptic transmission, which is essential for learning and memory. This project aims to explore how the mobility and nanoscale organization of AMPARs influence synaptic plasticity and information processing.
Research Objectives
- Investigate AMPAR Trafficking:
- Understand how AMPAR mobility and nanoscale organization control synaptic plasticity.
- Study the interaction of AMPARs with auxiliary proteins like TARP, CNIH, and Shisas.
- Develop and Apply Advanced Techniques:
- Combine electrophysiology on brain slices with light sheet imaging, optogenetics, and cell biology.
- Utilize innovative tools to control receptor mobility and subunit composition.
- Implement High-Resolution Imaging:
- Enhance Light Sheet Fluorescence Microscopy (LSFM) and Lattice Light Sheet Microscopy (LLSM) with adaptive optics for deep tissue imaging.
- Integrate Single Molecule Localization Microscopy (SMLM) techniques like U-PAINT and DNA-PAINT for nanometer-scale resolution imaging of AMPAR subunits.
Methodology
- Electrophysiology: Perform recordings on brain slices to analyze synaptic activity and plasticity.
- Light Sheet Imaging: Use LSFM and LLSM to visualize synapses in thick samples with minimal phototoxicity.
- Optogenetics: Control receptor dynamics using light-sensitive proteins to manipulate signaling pathways.
- SMLM Techniques:
- U-PAINT: Track individual AMPAR movements in rat hippocampal cultures.
- DNA-PAINT: Achieve high-resolution imaging of GluA1 and GluA2 subunits in organotypic brain slices.
Innovations and Expected Outcomes
- Adaptive Optics Integration: Correct optical aberrations in LLSM for clearer imaging.
- Advanced Marking Techniques: Develop robust methods for deep SMLM imaging in brain slices.
- Intracellular Signaling Control: Integrate photoswitchable kinases and phosphatases to study receptor dynamics.
Student Opportunities
- Hands-on Experience: Gain practical skills in electrophysiology, advanced microscopy, and molecular biology.
- Cutting-edge Research: Contribute to groundbreaking studies on synaptic plasticity and receptor dynamics.
- Collaborative Environment: Work with a team of experts in neuroscience and cutting-edge imaging technologies.
Join us in exploring the intricate world of synaptic transmission and plasticity, and make a significant impact on our understanding of brain function and diseases.
To apply, please send complete CV, letter of motivation and up to two letters of recommendations: daniel.choquet@u-bordeaux.fr
2024-05-27 (GROC Team) : NMDA receptor autoantibodies primarily impair the extrasynaptic compartment, BRAIN - May 24
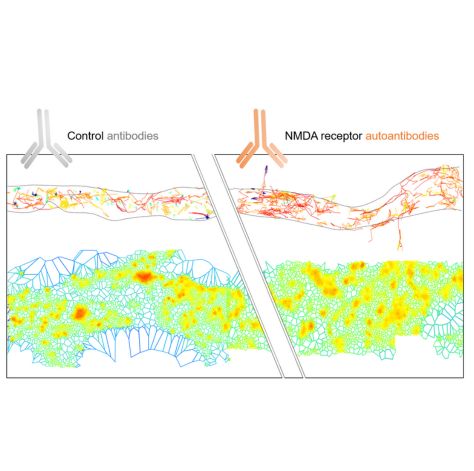
Zoe Jamet, Camille Mergaux, Morgane Meras, Delphine Bouchet, Frédéric Villega, Jakob Kreye, Harald Prüss, Laurent Groc
Brain. 2024-05-17
DOI : https://doi.org/10.1093/brain/awae163
Summary
Autoantibodies directed against the N-methyl-D-aspartate receptor (NMDAR-Ab) are pathogenic immunoglobulins detected in patients suffering from NMDAR encephalitis. NMDAR-Ab alter the receptor membrane trafficking, synaptic transmission and neuronal network properties, leading to patients’ neurological and psychiatric symptoms. Patients often have very little neuronal damage but rapid and massive (treatment-responsive) brain dysfunctions related to unknown early mechanism of NMDAR-Ab. Our understanding of this early molecular cascade remains surprisingly fragmented.
Here, we used a combination of single molecule-based imaging of membrane proteins to unveil the spatio-temporal action of NMDAR-Ab onto live hippocampal neurons.
We first demonstrate that different clones of NMDAR-Ab primarily affect extrasynaptic -and not synaptic- NMDAR. In the first minutes, NMDAR-Ab increase extrasynaptic NMDAR membrane dynamics, de-clustering its surface interactome. NMDAR-Ab also rapidly reshuffle all membrane proteins located at the extrasynaptic compartment. Consistent with this alteration of multiple proteins, NMDAR-Ab effects were not mediated through the sole interaction between NMDAR and EphB2 receptor. At the long-term, NMDAR-Ab reduce NMDAR synaptic pool by slowing down receptor membrane dynamics in a cross-linking independent manner. Remarkably, exposing only extrasynaptic NMDAR to NMDAR-Ab was sufficient to produce their full-blown effect on synaptic receptors.
Collectively, we demonstrate that NMDAR-Ab first impair extrasynaptic proteins, and then the synaptic ones. These data shed thus new, and unsuspected, lights on the mode of action of NMDAR-Ab and likely to our understanding of (extra)synaptopathies.
2024-05-13 (SIBARITA Team) : Job offer: engineer to develop light-sheet microscopy for neurosciences applications
Project description
Light Sheet Fluorescence Microscopy technics (LSFM) has demonstrated to be a method of choice for 3D imaging biological samples at various spatial and temporal scales with minimal photo-damaging effects.Several solutions have been developed in the field of neuroscience to image biological samples ranging from fixed whole brains,to single dissociated neurons growing on a coverslip. In this regard, the Interdisciplinary Institute for Neuroscience (IINS)and the Bordeaux Imaging Center (BIC)are equipped with 3complementary LSFMtechniques: (1) an ultramicroscope for whole brain imaging; (2) a Lattice Light Sheet Microscope(LLSM)to image the first layers of brain slices at high spatial resolution; (3) a single objective selective plane illumination microscope (soSPIM)dedicated to 3D cell cultures and in-depth single-molecule localization microscopy (SMLM).
We aim to complete our catalog by implementing a solution based on the Oblique Plane Microscopy (OPM) architecture, which will be dedicated to fast neuronal sample imaging, ie. brain slices,equipped with local photo-manipulation.
Missions
The candidate missions will be:
- to assemble a custom OPM to address specific neurobiological questions,
- to develop the instrument control software and iii) to participate in the testing and optimization of the OPM in close collaboration with neuroscientists.
Candidate profile
We seek for an independent, motivated and enthusiastic candidate, with an interest in neuroscience and a strong expertise in optics and fluorescence microscopy. Strong interest and good skills in programming for instrumentation are required.The candidate will work in an English-speaking environment, in close interactions with the neuroscientists’ team of the Bordeaux’s Neurocampus.
Environment
The candidate will be hosted in the Quantitative Imaging of the Cell team, a R&D team with an internationally-recognized expertise live cell microscopy and quantitative analysis.The Bordeaux Imaging Center, where the final instrument will be eventually transferred,is an imaging platform with a department dedicated to photonic microscopy for biology. It is equipped with several advanced fluorescence microscopy systems (Confocal, STED, SMLM, LLSM, ...). The BIC and IINS are hosted in the same building, a brand new neuroscience research center located on the Carreire campus of the Bordeaux University.
The Interdisciplinary Institute for Neuroscience (IINS) is an international level research center in neurosciences. It gathers 14 teams with complementary and interdisciplinary expertise, as well as several platforms to address cutting-edge questions in various aspects of neurosciences.
Contract
A 1 year, renewable, engineer position is available in the framework of the French “Grands Programmes de Recherche” BRAIN awarded to the Bordeaux Neurocampus. Applicants should send a CV, a motivation letter and contact details for at least two referees to:
jean-baptiste.sibarita@u-bordeaux.fr; remi.galland@u-bordeaux.fr; mathieu.ducros@u-bordeaux.fr
2024-03-27 (PERRAIS Team) : The team received an Equipe FRM award for their "SpaceNeuroModule" project

Spatio-temporal control of catecholamine neuromodulation of hippocampal pyramidal cells: from mechanisms of neuromodulator release to receptor signaling and trafficking (SpaceNeuroModule)
Scientific abstract
Chemical neurotransmission has been classified into fast neurotransmission and neuromodulation. Fast chemical neurotransmission, used by a majority of neurons in the central nervous system, is spatially restricted, thanks to a tight spatial control of neurotransmitter release at the active zone, the presence of cognate post-synaptic receptors and powerful recapture systems. In contrast, neuromodulation arises from a small number of neurons mediating slow communication to widespread neuronal networks. The sparsity of neuromodulatory synapses and the absence of direct ionotropic receptor signaling hampered our ability to resolve neuromodulation mechanisms at the micrometer and millisecond scale. In the project SpaceNeuroModule, we will address this question at noradrenaline projections in the hippocampus. We will use state-of-the art synaptosome sorting, proteomics, correlative cryo light and electron tomography (cryoCLEM), receptor interactome profiling, live cell fluorescence imaging and electrophysiology in vitro and ex vivo. We have defined 2 work packages to address the release of neuromodulators and the activation of their receptors at pyramidal dendrites, which regulates attention related learning and stress-related behaviors. First, we will determine the organization and dynamics of noradrenaline release sites. Second, we will assess the spatio-temporal extent of beta-adrenergic signaling in post-synaptic compartments. This research program will enable the formulation of design principles for neuromodulatory systems which will be tested further in disease models.
Lay abstract
The brain is composed of neurons which connect each other through synapses. A neuron sends neurotransmitter molecules to a connected neuron which captures them with specific receptors. Because a neuron receives up to thousands of synapses, some using the same neurotransmitter, each message must be sent precisely at the right place and travel minimal distance to the connected neuron and not spill over to neighboring synapses. Classical synapses are very specialized in ensuring this specific transmission with precise locations for release sites and receptors: decades of intense work from researchers around the world have discovered many of the building blocks of these synapses. A special category of neurons called neuromodulatory neurons, which comprise in particular dopaminergic neurons which degenerate in patients suffering from Parkinson’s disease, act in a different way. They do not mediate a fast signal but modulate large assemblies of neurons, even though they send molecules, in this case dopamine, to other neurons from specific sites. Because of technical difficulties such as the rarity of these neurons and their lack of fast signaling, we do not yet know how exactly is dopamine released and if specific sites are privileged, nor how receptors which are activated locally can convey the signal to the rest of the neuron. We will use state-of-the-art methods to purify neuromodulator synapses and identify new specific molecules (proteins). We will watch the structure of these sites with high resolution microscopes to understand what are the differences with classical synapses. Finally, we will image in brain tissue the release of neuromodulator and the activation and movement of their receptors to define how neuromodulation is organized and controlled in the brain.
2024-03-27 (TAKAHASHI Team) : The team received an Equipe FRM award
 Project: Cortical mechanisms for tactile information processing during tool use
Project: Cortical mechanisms for tactile information processing during tool use
Cortical mechanisms for tactile information processing during tool use
Scientific abstract
Tool-mediated tactile sensing is a crucial aspect of daily life. For instance, when using a fork, we can extract the food's location and texture as if the fork were part of our body. Similarly, a blind person uses a cane to perceive their surroundings and accurately estimate object locations. The brain incorporates tools like forks or canes into the “body schema” – the internal representation of our own body and its surroundings, which can be expanded by tools. This schema is believed to be maintained and constantly updated in the frontoparietal cortical network, enabling the brain to interpret somatosensory information and create a coherent tactile experience and perception of the surrounding space. However, the specific neuronal processes involved are largely unexplored.
Given the unique nature of rodent whiskers, non-neuronal, extra-somatic elements, we propose using the mouse whisker system, as a new model to study neural mechanisms in tool-mediated object localization. Furthermore, our research takes a novel approach with “prosthetic whiskers,” artificial whiskers that substitute a mouse’s innate whiskers, offering a unique opportunity to examine neuronal processes in the context of “extrinsic” tool use in mice.
The proposed study aims to test the hypothesis that the layer 1 of the primary somatosensory cortex, receiving feedback from the frontoparietal network, integrates body schema and somatosensory information during tool use. Utilizing advanced imaging and genetic manipulation, we will delve into neuronal processes from subcellular to network levels, as mice employ innate or prosthetic whiskers for object localization. This research would revolutionize our understanding of the neurophysiological underpinnings of tactile processing during tool use.
Lay abstract
How does the brain process tactile information through hand-held tools? While eating with a fork, the position and texture of the food is felt at the tips of the fork rather than at hand. Although the sensory input in this example is received at the hand surface, the tactile sensation of the food is created in the body space extended by the fork. Despite the prevalence of tool-based tactile sensing in daily life, the underlying neuronal processes remain largely unknown. This gap is due in large part to the fact that most of the neurophysiological studies of tool use are conducted in humans and monkeys, where only limited techniques and tools can be applied. To overcome this limitation, for this study, we will use mice, an animal model unconventional for the tool-use experiment. Mice have whiskers as innate tools, and we have recently developed artificial "prosthetic whiskers" that can replace them. By monitoring and manipulating neuronal activities with cellular and subcellular resolution, we will decipher the neuronal underpinnings that regulate tactile sensing during tool use. Our primary target is the layer 1 of the primary somatosensory cortex, where feed-forward somatosensory information is integrated with feedback information from higher-order cortical areas implicated in tool use. By taking full advantage of state-of-the-art techniques available for mouse research, the proposed study seeks to reveal the neurophysiological mechanisms of tactile processing during tool use in unprecedented detail. The insights gained would significantly enhance our understanding of the neural underpinnings of tool-based tactile sensing. Moreover, this research may have implications for other research areas, for example, paving the way for the design of neuroprosthetics with enhanced tactile sensing capabilities.
2024-03-18 : Activity Report 2023

The Insterdisciplinary Institute for Neuroscience's very first activity report is out! The entire IINS community is proud of the work accomplished in 2023 and proud to present it to you.
Discover our key figures and highlights for 2023!
Contents:
- IINS in a few figures
- A look back at 2023
- Success stories
- Translational successes
- Technological successes
Thank you to everyone who contributed to the production of this activity report. We hope you enjoy reading it!
2024-03-04 (ROUX Team) : 1st article from the team in Nature Communications
 Endogenous cannabinoids in the piriform cortex tune olfactory perception, Nat Com - Feb 24
Endogenous cannabinoids in the piriform cortex tune olfactory perception, Nat Com - Feb 24
Endogenous cannabinoids in the piriform cortex tune olfactory perception, Nat Com - Feb 24
Geoffrey Terral, Evan Harrell, Gabriel Lepousez, Yohan Wards, Dinghuang Huang, Tiphaine Dolique, Giulio Casali, Antoine Nissant, Pierre-Marie Lledo, Guillaume Ferreira, Giovanni Marsicano & Lisa Roux
DOI: 10.1038/s41467-024-45161-x
Summary
Whether and how the endogenous activity of cannabinoid type-1 receptors impacts sensory functions in vivo is largely unknown. Here, authors show - using in vivo electrophysiology, fiber photometry, behavioral assays and pharmacology - that it impacts network dynamics in piriform olfactory cortex and the ability of mice to detect odorants.
- How was the Roux team involved in this publication?
The team performed in vivo electrophysiological recordings in both freely moving and head-fixed conditions and conducted some behavioral assays. This work benefited from fruitful collaborations within the Neurocampus (Marsicano Lab and Guillaume Ferreira) and with the Pasteur Institute.
2024-01-04 (ROUX Team) : Evan Harrell becomes a CNRS researcher in the Roux team!

Evan Harrell joined IINS in 2021 as part of Lisa Roux’s team. In 2023, he passed the CNRS Section 25 competition and was awarded a CNRS researcher position from January 2024. Thanks this new position, Evan Harell will now be able to continue his research on the neural circuits by which olfactory experience leads to approach or avoidance behaviors within the Lisa Roux team.
What is your background?
"I studied in 3 different countries, earning a bachelor’s degree in Biomedical Engineering from Duke University (USA, 2005), followed by Masters’ degrees in Biomedical Engineering from the University of Oxford (UK, 2008) and Interdisciplinary Life Science from the University of Paris Descartes (France, 2009). For my PhD (2009-2014), I joined Gero Miesenböck’s lab (Centre for Neural Circuits and Behaviour) at Oxford (UK) where I identified some of the molecular machinery that supports homeostatic synaptic plasticity in the Drosophila olfactory system. As a post-doc (2015-2021), I returned to Paris to study olfactory and tactile sensory processing in the teams of Brice Bathellier (Institut de l’Audition) and Daniel Shulz (Neuro-PSI)."
Why did you join IINS?
"As a post-doc, I was more and more impressed by the ease with which smells are remembered by both humans and mice. The Roux laboratory at IINS was conceived to study this phenomenon, and it turned out that many of my friends and colleagues knew Dr Lisa Roux and recommended that I contact her about joining the team. After an official visit in 2019, it was clear to me that the dynamic and interdisciplinary environment at IINS would be an ideal place to achieve my research ambitions. I officially joined the Roux team in 2021 as a pre-recruitment chair at the University of Bordeaux and in 2023, I passed the CNRS section 25 concours to become a permanent researcher starting in January 2024."
Can you tell us about your research?
"Survival behaviors like evading predators or finding food and mates can be reduced to a series of simple decisions: to approach or to avoid? Scents play a very informative role in these choices, and my research aims to determine the neural circuit basis by which olfactory experience leads to learned approach or avoidance. To understand how odor attractions and aversions are learned and drive action, I am using in vivo two-photon microscopy and optogenetics during head-fixed or freely moving odor-driven memory tasks in mice."
Which difficulties have you encountered for have this new position?
"IIt was a long and winding path. After reaching out to Dr Roux in 2018, it took 6 years to secure my long-term position in the Roux team. The competition for permanent positions in France is fierce with many highly qualified candidates, and many auditions along with a long distance family life made these past years very challenging."
Which opportunites will offer to you this new position?
"I am thrilled to have finally secured my future at the IINS in the Roux team. As a permanent researcher, I can develop long term projects like an olfactory virtual reality where mice navigate in a virtual world defined only by smells. On fixed term contracts, I was always faced with uncertainty over my future, whether from doubt about an upcoming contract extension or impending evaluation. This constant pressure forced me to focus solely on short and middle term prospects. Now, I feel much more relaxed and am free to engage in challenging projects that can have a much bigger impact."
Any advice for scientist and in particularly to a scientist who are looking for a researcher position?
"For young scientists, I think the simplest personal advice is patience and persistence. Do not let rejection demoralize you. Listen to the feedback, improve your file, and try again. From a scientific standpoint, I think the key to success in the current research landscape is collaboration. The work that I am most proud of in my career has come from fruitful interactions with fellow scientists who have broadened my perspectives and forced me to be more deliberate in presenting our results."
2023-10-23 (MULLE_CARTA Team) : GluK2 Is a Target for Gene Therapy in Drug-Resistant Temporal Lobe Epilepsy

*Boileau, C., *Deforges, S., *Peret, A., Scavarda, D., Bartolomei, F., Giles, A., Partouche, N., Gautron, J., Viotti, J., Janowitz, H., Penchet, G., Marchal, C., Lagarde, S., Trebuchon, A., Villeneuve, N., Rumi, J., Marissal, T., Khazipov, R., Khalilov, I., Martineau, F., Maréchal, M., Lepine, A., Milh, M., Figarella-Branger, D., Dougy, E., Tong, S., Appay, R., Baudouin, S., Mercer, A., Smith, J.B., Danos, O., Porter, R., #Mulle, C., #Crépel, V., 2023. Ann Neurol. doi:10.1002/ana.26723, *co-first authors, # co-senior authors
Summary
The teams of Christophe Mulle (CNRS researcher and IINS Team Leader, University of Bordeaux) and Valerie Crepel (INMED, Inerm, Marseille) have demonstrated that localized injection of a viral vector targeting GluK2/GluK5 kainate receptors in the hippocampus is a highly promising gene therapy strategy for controlling seizure onset in drug-resistant epilepsy patients. Based on the gene therapy strategy described in the Annals of Neurology article, the Dutch company uniQure, which has acquired the start-up Corlieve Therapeutics - of which Valerie Crepel and Christophe Mulle were scientific co-founders - will launch a Phase I/IIa clinical trial in the USA to for the treatment of drug-resistant temporal lobe epilepsy.
Presentation of the article
This article is the fruit of a long-standing collaboration between Christophe Mulle and Valerie Crepel. It represents a key step in the development of a gene therapy approach for the treatment of temporal lobe epilepsy. Epilepsy, which consists in the synchronized, abnormal excitation of a group of neurons in the cerebral cortex, affects around 600,000 people in France. Temporal lobe epilepsy is the most common form of epilepsy in adults, with the affected area mainly in the hippocampus. Medical treatment, using antiepileptic drugs to normalize the hyperactivity of cortical circuits, is effective in 70% of cases. For drug-resistant forms, surgical resection is often the only option left. Against this backdrop, the two teams have developed a translational project targeting kainate-type glutamate receptors.
This project is based on the hypothesis that GluK2/GluK5-type kainate receptors localized ectopically at aberrant synapses formed by the sprouting of axons from dentate gyrus granule cells, act as detonators in triggering epileptic discharges in the hippocampus. In 2014, an initial study by these researchers demonstrated that the genetic deletion of grik2, the gene encoding the GluK2 subunit of the kainate-type glutamate receptors, or pharmacological inhibition of GluK2/GluK5 receptors, led to a reduction in the number of spontaneous and recurrent seizures observed in a mouse model of temporal lobe epilepsy. A patent had been filed indicating GluK2/GluK5 as a potential target for treating temporal lobe epilepsy.
In the "Annals of Neurology" article, the teams of Valerie Crepel and Christophe Mulle (and in his team the work carried out mainly by Severine Deforges, IR CNRS), chose to develop a strategy based on interfering RNAs to reduce grik2 gene expression locally in the hippocampus. The article describes the strategy for selecting interfering RNA sequences in cell models, and the production of a viral vector that enables expression of an anti-grik2 microRNA in hippocampal neurons. This viral vector was injected in vivo into epileptic mice. The article demonstrates the efficacy of this viral vector in decreasing grik2 expression and GluK2 levels in the hippocampus, and ultimately in reducing epileptic activity in mice in vivo. Moreover, administration of the viral vector to organotypic hippocampal slices obtained from surgically resected epileptic patients, led to the suppression of epileptiform discharges recorded in these slices.
In conclusion, localized injection of a viral vector targeting grik2 expression in the hippocampus represents a highly promising gene therapy strategy for controlling seizures in drug-resistant epilepsy patients.
The work described in this article goes hand in hand with an industrial drug development strategy that Valérie Crépel and Christophe Mulle have been pursuing for several years, with the support of Aquitaine Science Transfert, Inserm Transfer and the Kurma Partners investment fund. Valerie Crepel and Christophe Mulle were the scientific co-founders of start-up Corlieve Therapeutics in October 2019, a company that was acquired in July 2021 by uniQure, a Dutch company specializing in the development of gene therapy for neurological diseases.
In September 2023, in parallel with the publication of the article in Annals of Neurology, uniQure issued a press release (see attached pdf), announcing that “the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for AMT-260, the Company’s gene therapy candidate for refractory mesial temporal lobe epilepsy (MTLE).” “AMT-260 comprises an AAV9 vector that locally delivers two engineered miRNAs designed to degrade the GRIK2 gene and suppress the aberrant expression of glutamate receptor subtype GLUK2 that is believed to trigger seizures in patients with refractory MTLE.”. Accordingly, following the gene therapy strategy described in the Annals of Neurology article, uniQure will launch a Phase I/IIa human clinical trial to be conducted in the US in early 2024.

Find out more about uniQure's press relea
2023-10-13 (ROUX Team) : Olfaction and Neurosciences Symposium 2023
 Exciting event on October 16, 2023
Exciting event on October 16, 2023
Event co-organized by Lisa, Guillaume Ferreira and Giovanni Marsicano on October 16, 2023 with the support of the Neurocampus Department and the IdEx. Check out the program!
2023-10-13 (ROUX Team) : Evan Harrell obtained a researcher position at the CNRS and is joining the team!
The team is happy to welcome a new permanent member.
Evan will bring complementary expertise to the team such as calcium imaging, Neuropixel recordings and computational approaches. His main project will focus on valence coding in olfactory circuits. Congratulation to him!
2023-09-25 (GROC Team) : The PHRC TiM-DepisT project

The PHRC TiM-DepisT project is a national hospital-based clinical research project. Its aim is to detect (DepisT) and treat (TiM = immunomodulatory therapy) the presence of autoimmunity in people suffering from psychiatric disorders (psychosis).
Led by Frederic Villega (CHU Bordeaux/IINS) and involving 9 University Hospitals across France, the project involves psychiatrists, child psychiatrists, neuropaediatricians and researchers. Psychiatrists Marion Leboyer from the Fondamental Foundation, Bruno Aouizerate, David Misdrahi, Sebastien Gard and Anouck Amestoy from Bordeaux Charles Perrens CHU, will be working hand in hand with researchers from Laurent Groc's IINS team (Delphine Bouchet, Olivier Nicole, Julien Dupuis, Helene Grea) and the Bordeaux CHU immunology department team (Isabelle Pellegrin, Patrick Blanco, Cecile Bordes).
The project was born in 2019 thanks to collaborative work between the research team of Laurent Groc (CNRS/University of Bordeaux), and the neuropaediatrician Frederic Villega, with the support of the Clinical Research Department of Bordeaux University Hospital.
In terms of funding, the project was supported by the the French government with the “Direction Generale de l'Offre de Soins”. The strength of this project lies both in the clinical question and in the fundamental and technological advances made by the IINS research laboratory. IINS researchers have built a diagnostic platform, coordinated by Delphine Bouchet (CNRS Research Engineer). It enables the presence of pathological autoantibodies to be detected with a high degree of reliability, which is a fundamental issue in this field. This effort began more than 10 years ago with the support of the “Fondation de le Recherche Medicale” (FRM), the Fondation “FondaMental”, and more recently, thanks to the award in 2019 of the SPARK prize from the University of Bordeaux. Thanks to the emergence of this innovative platform, the clinical project can now be rolled out with a budget of one million euros.
The project has also had a long road ahead, with submissions to many institutions (“Agence nationale de securité du medicament", ethics committees, etc.) and the covid pandemic. Nevertheless, diagnosis and treatment of the disease will be able to begin in October 2023.
The prospects for the PHRC TiM-DepisT project are therefore significant. Between 2024 and 2027, researchers plan to carry out diagnostic research on 1,000 patients. The aim is to detect and provide access to immunotherapy treatment for several dozens of them. This unprecedented system opens up a host of possibilities for the benefit of medicine, which is in the midst of a revolution as a result of the discovery of the multiple implications of autoimmunity.
Frederic Villega has a few words to say about the PHRC TiM-DepisT project :
"As a clinician at the CHU, who has been working with Laurent Groc and his team for many years, I have structured this bridge between the clinical and the technological. This enables us to transfer elements of major fundamental discoveries for the benefit of healthcare! In this way, I've been able to bring together the strengths of Bordeaux University Hospital and the IINS to build an incredibly ambitious project that will provide answers to major burning questions about the new concept of immuno-psychiatry. [...] Laurent Groc was the researcher who, with his team and his international partners, climbed the first steps of an immense edifice concerning the physiopathology of psychiatric illnesses. This project represents immense hope for all mankind. It is, of course, one of many examples of the importance of research to healthcare and the quality of life of the men and women of tomorrow. [...] The aim of this research project is to answer 2 major questions in the context of mental health care: How prevalent is dysimmunity among the many factors involved in the pathophysiology of the diagnosis of psychosis? And to what extent can these patients, with an autoimmunity, respond to immunomodulatory therapy? This project could prove to be a major global turning point in psychiatric diagnosis and treatment, offering millions of families the hope of a brighter future!
2023-09-25 (CHOQUET Team) : 2 days of exciting science on synapse biology around the PhD defense of Agata Nowacka
 Seminar Molecular and cellular mechanisms of synapse physiology
Seminar Molecular and cellular mechanisms of synapse physiology
Monday 6th November 2023
Workshop: Virtual Presynaptic Terminal
We invite you to a workshop where a collaborative team from University College London (UCL) and the University of Warwick will introduce a computational modelling framework that enables in silico exploration of the mechanisms of Ca²⁺-driven neurotransmitter release in different synapses.
Morning session 9:00 – 12:30
• Kirill Volynski: Introduction to the modelling framework with examples linking the model and experimental data.
• Yulia Timofeeva: 3D modelling of presynaptic Ca2+ dynamics, covering Ca2+ influx, buffering, and diffusion.
• Chris Norman: Modelling Ca 2+ activation of vesicular release and short-term synaptic plasticity.
• Round table: model expansion across the synaptic cleft.
Afternoon session 14:00 – 17:00
• Yulia Timofeeva and Chris Norman: hands-on training for application of the virtual presynaptic terminal model. Contact the presenters to book a one-to-one training session.
Registration before October 20: https://evento.renater.fr/survey/workshop-virtual-presynaptic-terminal-iyqqwh9v
Tuesday 7th November 2023
Seminar Molecular and cellular mechanisms of synapse physiology
Venue: Centre Broca Nouvelle-Aquitaine (Amphitheater Broca)
Program
9h30 - Maria Passafaro "Unraveling PCDH19-related pathogenic mechanisms in Developmental and Epileptic Encephalopathy 9 (DEE9)"
10h10 - Andrea Barberis 'Spatial determinants for the interactions of glutamatergic and GABAergic synapses in dendrites of hippocampal pyramidal neurons"
10h50 - Kirill Volynski "Synergistic regulation of neurotransmitter release by different synaptotagmin isoforms"
11h30 - Aude Panatier "Astrocytic EphB receptors control NMDAR functions and memory"
14h - PhD defense of Agata Nowacka "Differential contributions of pre- and postsynaptic components in tuning high-frequency short-term synaptic plasticity"
Contact
Kirill Volynski (k.volynski@ucl.ac.uk), UCL Queen Square Institute of Neurology
Chris Norman (c.norman@ucl.ac.uk), UCL Queen Square Institute of Neurology
Yulia Timofeeva (y.timofeeva@warwick.ac.uk), Department of Computer Science, University of Warwick
2023-09-14 : Welcome to the Interdisciplinary Institute for Neuroscience, IINS

Put yourself in the shoes of an IINS scientist and find more about our research topic!
Created in 2011 by the CNRS and the University of Bordeaux, IINS is a research center that investigates brain cell communication in the healthy and diseased brain. The Institute is located on the Bordeaux Neurocampus, near to the city center. This is an open place in which we welcome scientists from around the world as well as the General Public. Exciting discoveries and ideas are discussed throughout the year in a large number of conferences and symposium.
With nearly 200 scientists, our goal is to shed light on the fundamental molecular mechanisms underpinning brain cell communication in health and disease, aiming at paving the way for innovative therapies for major neurological and psychiatric diseases. Together, we are working on multidisciplinary projects while highlighting the development of new technologies and tools.
In a few figures, IINS counts approximately 60 publications per year, 50 international collaborations, 10 ERC grants and several patents!
At the forefront of brain research, IINS scientists decode how neuronal networks compute information from the nanoscale to the behavior level. It's a stimulating environment for academic research and biotech companies.
What about you? What are you waiting for to join IINS?!
Follow us on social networks:
- X: https://twitter.com/iins_bordeaux
- LinkedIn: https://www.linkedin.com/company/interdisciplinary-institue-neuroscience-bordeaux-iins
- YouTube: https://www.youtube.com/@iins_bordeaux
2023-09-14 (CHOQUET Team) : #IMadteIt: Marie-Lise Jobin, a post-doctoral researcher starting out as an associate professor

Marie-Lise Jobin is currently a post-doctoral researcher in Daniel Choquet’s "Dynamic organization and function of synapses" team. Interested in the role of the cytoskeleton in the function and structure of dendritic spines, the scientist is supervised by Anna Brachet. Marie-Lise recently obtained a permanent teaching-research position at the Institut National Polytechnique de Bordeaux (Bordeaux INP). Here, she looks back on her career.
What is your background?
"I studied biology and structural biochemistry at the University of Bordeaux. During my Master’s and PhD, I worked in many different laboratories. First, I spent three months working at the University of Guelph in Ontario, Canada. Then, for my PhD, I joined the Institute of Chemistry and Biology of Membranes and Nano-objects (CBMN) in Pessac. On completion of my studies, I spent a year as a post-doctoral fellow at the Nutrition and Integrative Neurobiology Laboratory (Nutrineuro) in Bordeaux. I then spent over three years at the University of Würzburg in Bavaria, Germany. And for the last four years I’ve been at IINS. My post-doctoral adventure will finally come to an end at the end in September 2023, and I’ll be taking up a position as an associate professor in September!"
Why did you join IINS?
"Generally speaking, I’ve always been interested in biomolecular interactions involving cell membranes. I first worked on synthetic membrane models. However, I’ve always wanted to study more physiological systems. So the neuroscience field allows me to work with systems closer to physiology, and to use my highly interdisciplinary skills. [...] Anna Brachet’s field of research on the role of cytoskeletal proteins, in particular spectrins, and their partners, in the regulation, function and mechano-transduction of dendritic spines fit perfectly my research interests. Moreover, IINS is considered one of the most recognized institutes in the field of neuroscience. Above all, IINS is part of a synergistic system, with close relations to the Bordeaux Imaging Center (BIC). It was a unique opportunity to develop my skills in cutting-edge microscopy techniques and gain access to considerable knowledge in this field!"
Can you tell us about your research?
"I’m trying to understand the role of cytoskeletal proteins, beta spectrins, in neurons, and more specifically in dendritic spines, which are the seat of neuronal transmission. My results showed that beta spectrins are co-organised on a nanoscopic scale in dendritic spines and are particularly important for the stability of these structures. Their membrane or cytoskeleton partners are all the more important in maintaining and stabilising these spectrins under the membrane and influence their nano-organisation."
As a woman in science, what difficulties have you encountered?
"My biggest difficulty has always been a lack of self-confidence. But throughout my career I’ve been lucky enough to meet scientists, men and women who have had faith in me, and who have always supported and encouraged me along the way."
Why do women need to be more recognised in the scientific community?
"It seems quite logical to me that gender diversity, or diversity of any kind, leads to better, more creative and innovative science. I can see that things are changing, slowly but surely, in the right direction, and I’m optimistic for the future!"
Any advice for young researcher?
"I’m proud to have got where I am, to have succeeded in obtaining a permanent position in academia, and to have stayed the course despite many years of not knowing what would happen and never giving up. You have to persevere. It’s often the fear of failing that stops us from moving forward, so don’t hesitate, dare to take the plunge and, above all, trust yourself."
2023-08-28 (CHOQUET Team) : Daniel Choquet is a member of the 2023 Fulbright France

Created in 1946 at the proposal of the eponymous US senator, Fulbright is an international academic mobility program of the US government. The aim of the program is to promote educational and cultural exchanges between France and the United States.
Since its launch, 21,244 students from both countries have benefited from the program:
- 12,294 French students have gone to the United States of America,
- 8,950 Americans have come to France.
Daniel Choquet (director of research at the CNRS, director of IINS, the BIC, the cluster of excellence BRAIN_2030 and team leader at IINS) has just been selected as a laureate of the Fulbright France program. He will be spending a 7 month sabbatical stay in Denver in 2024.
Why did you apply to the Fulbright France Program?
“I was getting organized to perform a 7 month sabbatical in Denver in 2022, in the lab of Pr. Mark Dell’Acqua. Given the cost of living in the US, I needed additional funding to support my stay. The Fulbright organization is a very prestigious academic mobility program of the US government and it was thus natural for me to apply, particularly given that there is a specific partnership with our region Nouvelle-Aquitaine.”
What does it mean to you?
“Well, it does mean a lot. First, in searching the Fulbright alumni file, I found out that both my parents were fulbrighters in the 50s while they did post-docs in Princeton, my mother with Albert Einstein. Second, I realized when I obtained this fellowship that when you enter a new family, the family of Fulbrighters, beyond the money, you enter a support network all over the US that will be both very interesting and valuable.”
Will you take this opportunity to discover or develop a research topic?
“Absolutely, the Department of Pharmacology at the University of Colorado in Denver that I’ll join hosts a large number of high profile neurobiologists that develop orginal cell biology approaches.
We will join our forces with Mark Dell’Acqua and Matthew Kennedy to develop new methods and approaches to control receptor trafficking and visualization.”
What do you think this program will bring you, both professionally and personally?
“In addition to the funding that will help me live there, being a Fulbright fellow in the US is really recognized and will help me develop my network and meet many academics in various disciplines. Fulbright fellow also have a mission to be ambassadors of their country, and I certainly intend to introduce to colleagues to some French cuisine specialties!”
2023-06-20 (THOUMINE Team) : Astrocyte Calcium Signaling Shifts the Polarity of Presynaptic Plasticity, Neuroscience - June 23
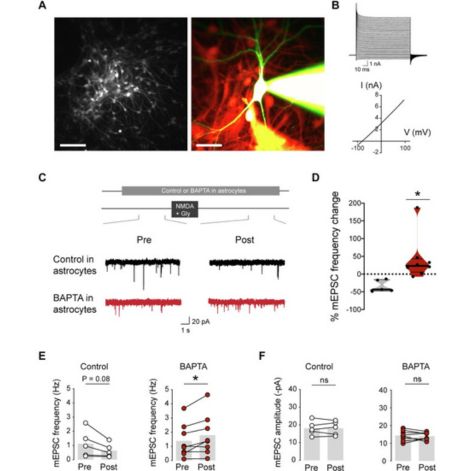
Mathieu Letellier, Yukiko Goda
Neuroscience. 2023-06-07
DOI: https://doi.org/10.1016/j.neuroscience.2023.05.032
Summary
Astrocytes have been increasingly acknowledged to play active roles in regulating synaptic transmission and plasticity. Through a variety of metabotropic and ionotropic receptors expressed on their surface, astrocytes detect extracellular neurotransmitters, and in turn, release gliotransmitters to modify synaptic strength, while they can also alter neuronal membrane excitability by modulating extracellular ionic milieu. Given the seemingly large repertoire of synaptic modulation, when, where and how astrocytes interact with synapses remain to be fully understood. Previously, we have identified a role for astrocyte NMDA receptor and L-VGCC signaling in heterosynaptic presynaptic plasticity and promoting the heterogeneity of presynaptic strengths at hippocampal synapses. Here, we have sought to further clarify the mode by which astrocytes regulate presynaptic plasticity by exploiting a reduced culture system to globally evoke NMDA receptor-dependent presynaptic plasticity. Recording from a postsynaptic neuron intracellularly loaded with BAPTA, briefly bath applying NMDA and glycine induces a stable decrease in the rate of spontaneous glutamate release, which requires the presence of astrocytes and the activation of A1 adenosine receptors. Upon preventing astrocyte calcium signaling or blocking L-type VGCCs, NMDA + glycine application triggers an increase, rather than a decrease, in the rate of spontaneous glutamate release, thereby shifting the presynaptic plasticity to promote an increase in strength. Our findings point to a crucial and surprising role of astrocytes in controlling the polarity of NMDA receptor and adenosine-dependent presynaptic plasticity. Such a pivotal mechanism unveils the power of astrocytes in regulating computations performed by neural circuits and is expected to profoundly impact cognitive processes.
2023-06-02 (MULLE_CARTA Team) : Christophe Mulle appointed "Chevalier de l’Ordre National du Merite"

Christophe Mulle is a research director at the CNRS and co-director of the "Synapse and neuronal circuits" team at IINS. A cellular neurobiologist, he specialises in glutamate receptors, in the electrophysiology of synaptic transmission and neuronal circuits. His research focuses on the plasticity of synaptic properties and neuronal circuits in the hippocampus, in the context of episodic memory encoding. Christophe Mulle also explores the mechanisms of synaptic dysfunction that occur in the context of Alzheimer's disease. At the same time, he is involved in a translational project to combat temporal lobe epilepsy using gene therapy, in close collaboration with Valerie Crepel. This has led to the creation of a start-up, Corlieve Therapeutics, of which he is scientific co-founder.
Christophe Mulle has invested a great deal of time in coordinating actions for the neuroscience research community in Bordeaux, as well as at a national and European level. For example, he has created the Bordeaux School of Neuroscience, which hosts the prestigious international Cajal training courses.
Christophe Mulle has been made a "Chevalier de l’Ordre du Merite" by the French Minister for Research. The award will be presented to him by Manuel Tunon de Lara, former President of the University of Bordeaux and himself a "Chevalier de l'Ordre du Merite", on Friday 2 June 2023.
Instituted by the General de Gaulle, "l’Ordre National du Merite" is the second national order after the Legion d'Honneur. Its purpose is to reward "distinguished merit" and encourage the country's driving forces.
Christophe Mulle has already won numerous awards. Nevertheless, all of them have a real meaning for the researcher.
What does this new award mean to you?
"It is an honour, of course, even if it was completely unexpected. It was Frederique Vidal, the former Minister for Research, who announced it to me in a letter in the middle of the Covid period. First of all, I looked into the award to find out what it was all about. In the end, of course, I am delighted!"
Why did you get it?
"I do not know who nominated me and why. Unlike a prize such as that the one from the Academie des Sciences, which rewarded the research I had carried out in the field of synapse biology, I suppose that this nomination bears witness to the role that I played in structuring neuroscience research in Bordeaux, and in promoting this discipline at both national and European level."
Any words for the scientific community?
"It is a real privilege to do the job we do. The role you can play in coordinating training and research structures is important and really satisfying! Nevertheless, I never lose sight of the fact that my job is to be a researcher. Driven by curiosity about how things work and what they are used for, it is an immense pleasure to come to the lab and discuss research issues with all my colleagues, the young and more established alike. And then, of course, to implement a wide range of projects. And at Bordeaux Neurocampus, a real bonus!"
2023-06-02 (GROC Team) : NMDA receptor functions in health and disease: Old actor, new dimensions, Neuron - May 23

Julien P. Dupuis, Olivier Nicole, Laurent Groc
Neuron. 2023-05-25
DOI: https://doi.org/10.1016/j.neuron.2023.05.002
2023-04-28 (SIBARITA Team) : #IMadeIt: Marine Cabillic, a woman scientist who knows how to adapt

Read the portrait of Marine Cabillic in English and French
Marine Cabillic, former research engineer in the team of Jean-Baptiste Sibarita, looks back on her five-six years at IINS. She is now software product manager at ONI (Oxford Nanoimaging) where she is responsible for a cloud software solution. At IINS, she has implemented several software solutions, analysis pipelines, as well as biology and imaging protocols for the automation of super-resolution microscopy by localisation
What is your background?
"I have always been interested in the health field. Thus, throughout my career, I have developed tools for the acquisition and analysis of biological or biomedical images. First, I joined ISEN Brest, an engineering school also has a speciality in biomedical technologies. Then, I did my last year of the master’s degree in a work-study program at Medimaps which is a start-up company specialised in medical imaging software. When I graduated, I joined Jean-Baptiste Sibarita’s team [...] and after two years at the Institute, I did a cifre thesis with Sanofi. This was part of a partnership between Sanofi-Aventis in Paris and the Institute. Then, at the end of my thesis and six months as a post-doc, I was hired at ONI."
Why did you join IINS?
"I initially had no expertise in neuroscience. However, I had a background in health and biotechnology, with software design. Moreover, the Sibarita team is a technical team where neuroscience is not the main subject of the research project. [...] For me, IINS is very interdisciplinary and innovative, with very varied projects, tools and fields of application! IINS is internationally recognised, which allows for more collaborations and projects. I joined IINS because I wanted to work in research and innovation. [...] At the Institute, in collaboration with Sanofi, I developed an automated method, combining high-content screening (HCS) and super-resolution, capable of screening and quantitatively characterising therapeutic antibodies for immunotherapy applications. The idea was to characterise the organisation and trafficking of antibody/therapeutic receptor pairs at the membrane of T cells in 96-well plates by quantitative single molecule localisation microscopy. This was done using the HCS-SMLM platform but I also worked on single cells using super-resolution multiplane light sheet imaging (soSPIM)."
As a woman in science, what difficulties have you encountered?
"I faced a lot of technical challenges during my thesis. At the beginning, being quite isolated in terms of a project that was not neuro-focused, I did not dare to go and ask other teams for advice. Also, when the project was not moving in the right direction, I found it difficult to confront the ideas of my collaborators. But the scientific world is very collaborative, to evolve in this world, you have to open up and exchange with others. So this what I did, I openned-up and ask for help of my peers to overcome my difficulties!"
Why do women need to be more recognised in the scientific community?
"In my field, which is technical, there are very few women. So it is more difficult to get ahead. In order to achieve gender equality, it is important to promote more women who carry out research projects or technical theses in order to reach young women as early as possible. Nevertheless, year after year, I notice an increase in the number of women working in technical research!"
Professional pride?
"I have very often embarked on new projects and fields. So I had to learn to adapt. During my thesis with Sanofi, I had to learn everything about biology. And I succeeded, I ended up developing protocols for culture, transfections, immune-labelling and imaging. Before that I had never touched a pipetboy in my life. When I accepted my current position at ONI, I also threw myself into the unknown, a position of responsibility, no initial skills, in English and working from home, big challenge but I adapted!"
Any advice for young researcher?
"Go for it. Why not yourself? First author of a paper, give a talk at that conference, win that prize, try a project, or apply for that kind of job,... Have fun. Science is fun, try projects/collabs, experiments, explore avenues. Train as much as you can and attend conferences,... Get organised. Planning your research projects is a key and important point that will avoid surprises and allow you to change your strategy if necessary and at the right time. Popularise your science! The first few times are hard but you will come out better."
2023-04-26 (SIBARITA Team) : Job offer: Engineer to automated fluidics and microfabrication for multi-conditions 3D biological
 Engineer to automated fluidics and microfabrication approaches for multi-conditions observation of 3D biological samples
Engineer to automated fluidics and microfabrication approaches for multi-conditions observation of 3D biological samples
The Sibarita team "Quantitative Imaging of the Cell" is seeking an engineer to develop automated fluidics and microfabrication approaches for multi-conditions observation of 3D biological samples using the soSPIM technology.
Project description
Spheroids and organoids have emerged in the last decade as very promising biological models for applications ranging from fundamental research to toxicology assays or drugs screening. However, the difficulties to culture and image them in 3D hamper their full adoption by laboratories and compagnies. In the meantime, Light Sheet Fluorescence Microscopy technics (LSFM) have proven to be extremely efficient for 3D imaging of biological samples at various spatial and temporal scales with minimal photo-damaging effects.However, LSFM technics are usually restricted in the number of sample and/or condition that can be probed due to complex sample mounting constraints. To address those questions, we develop in collaboration with V.Viasnoffand G. Grenci teams at MBI (NUS, Singapore) a culture and imaging platform combining microfabricated micro-wells,with a single-objective-based LSFM architecture named soSPIM. This combination allows to standardize and parallelize both the culture and the imaging of complex 3D biological models, paving the way toward the use of spheroids and organoids in multi-conditions screening experiments.
In that perspective,we aim to develop new culture vessels that would allow to transform our culture and imaging platform in a multi-condition one. Those new vessels will have to allow the appropriate and timely delivery of media and chemical compounds into the 3D cultured models. Then, a dedicated process will be implemented to allow the automated monitoring of those different conditions using the soSPIM 3D imaging technology.
Missions
The candidate missions will be:
- to develop a custom fluidics system for media and chemical compounds delivery into multi-wells plates,
- to adapt the fabrication process of the JeWell devices to this multi-well plate format. She/he will also participate to the validation of the multi-conditions systems created performing toxicology assays on 3D biological models developed in the team or by collaborators.
Candidate profile
We seek a motivated, enthusiastic and independent candidate, with a strong expertise in fluidics and automation and showing an interest in biology. Complementary skills in fluorescence microscopy, and/or programming would be appreciated. The candidate will work in an English-speaking environment, in close interactions with biologist in Oncology (BRIC).
Contract
Applicants should send a CV, a motivation letter and contact details for at least two referees to: jean-baptiste.sibarita@u-bordeaux.fr and remi.galland@u-bordeaux.fr
2023-04-21 (CHOQUET Team) : Regulation of different phases of AMPA receptor intracellular transport by 4.1N and SAP97, eLife
 April 2023
April 2023
Caroline Bonnet1, Justine Charpentier1, Natacha Retailleau1, Daniel Choquet1,2, Françoise Coussen1*
1University of Bordeaux, CNRS, Interdisciplinary Institute for Neuroscience, Bordeaux, France; 2Bordeaux Imaging Center, Bordeaux, France
eLife. 2023-04-20
DOI: https://doi.org/10.7554/eLife.85609
Françoise Coussen, Director of research at the CNRS at IINS worked on AMPAR intracellular transport and directed this work helped by Daniel Choquet. Caroline Bonnet, PhD student, performed the experiments helped by Justine Charpentier who performed all biochemistry experiments and by Natacha Retailleau (molecular biology).
Find the explanations of the scientists of this publication
Identification of the molecular mechanisms regulating the intracellular transport of glutamate receptors: a new pathway for controlling synaptic plasticity
"The modulation of the efficiency of synaptic transmission between neurons is one of the fundamental processes of memory and learning phenomena. This regulation of the strength of synaptic transmission is largely driven by changes in the number of receptors present at the synapse. In this work, the researchers identify a new mechanism for controlling the establishment of receptors at the level of the synapse through the control of their intracellular transport.
Neurotransmitter receptors, and in particular glutamate receptors, are concentrated in synapses in front of neurotransmitter release sites. However, in the process of their biogenesis, these receptors are synthesized at the level of the endoplasmic reticulum, most of the time several hundred microns from the synapses. They must therefore be transported to the synapses. Our previous work had made possible to visualize for the first time the intracellular transport of AMPA-type glutamate receptors, responsible for the majority of the rapid excitatory transmission between neurons. These receptors are transported rapidly (1-2 microns per second) in vesicles circulating on the microtubules using molecular motors. We observed that, surprisingly, this transport was strongly regulated by neuronal activity.
In this new work, we have identified the molecular mechanisms responsible for these regulations. The cytosolic C-terminal domain of the AMPAR GluA1 subunit is specifically associated with two proteins, 4.1 N and SAP97. We analyzed how interactions between GluA1 and 4.1N or SAP97 regulate GluA1 transport and its exocytosis under basal conditions and after induction of synaptic plasticity (LTP). Our results identify differential roles of 4.1N and SAP97 in controlling the different phases of transport and membrane integration of GluA1.
This work opens new perspectives in understanding the molecular mechanisms that control the establishment and maintenance of glutamate receptors at the synapse during synaptic plasticity."
2023-04-18 (GIANNONE Team) : Gregory Gianonne and his team win the FRM Team Price 2023

Created in 1948, the Fondation pour la Recherche Medicale (FRM) has as an aim to support and fund the public research in every medical and pathophysiologic fields.
Thus, the FRM supports more than 400 new researches conducted in the laboratories of public research and higher education organisations every year (INSERM, CNRS, INRA, CEA, Universities, Prestigious Universities, health institution, …).
Gregory Giannone (CNRS researcher and team leader “Mechano-biology of motile and neuronal structure”) and his team have just won the FRM Team Price 2023!
This price is awarded to teams proposing an innovative research program in biology with potential health applications.
Gregory Giannone’s team explains us their project:
"Adhesive and cytoskeletal structures control cell functions such as migration and proliferation. As such they regulate cell behavior during physiological processes such as development; but when altered, these structures contribute to pathologies including cancer. How assembly of adhesive and cytoskeletal structures and resulting mechanical forces are coordinated to shape cell movements and morphologies during development and cancer progression remains fundamental questions. Despite recent advances in imaging methods in multicellular environments (organoids, small organisms), a molecular understanding of these fundamental processes is still lacking. To reach this molecular understanding, we developed for the last ten years, new strategies to study integrin adhesions and actin-based protrusions at the molecular level using super-resolution microscopy and single protein tracking. We unraveled key molecular events leading to: integrins activation and mechano-sensing in healthy and cancer cells; actin assembly in dendritic spines, and in lamellipodia. However, these findings were obtained by studying isolated cells on stiff 2D substrates. In this proposal, we aim to reach a molecular understanding of cell movements and morphologies in 3D multicellular assemblies characterized by softer, confined and dynamic 3D environments. In this project, we will decrypt the mechanical and biochemical molecular rules that govern the assembly, dynamics and coordination of integrin adhesions and actin protrusions in 3D multicellular systems during two fundamental processes: (AIM 1) the formation of long-lasting macromolecular complexes in vivo during development of integrin-based muscle attachment sites in Drosophila; (AIM 2) the formation of transient macromolecular complexes supporting the ability of Small Cell Lung Cancer cells to assemble into spheroids and to migrate during metastasis. This project could help to find new and more specific therapeutic strategies against this cancer."
Congratulations to the "Mechano-biology of motile and neuronal structure" team!
2023-04-05 (THOUMINE Team) : Portrait of Mathieu Letellier who receives the CNRS 2023 bronze medal

Read the portrait of Mathieu Letellier in English and French
Mathieu Letellier is a CNRS researcher in Olivier Thoumine’s team "Cell Adhesion Molecules in Synapse Assembly” part of the IINS. For many years, he has been interested in the processes that control the plasticity and development of neural connections. His work has highlighted the role of adhesion proteins and neuronal activity in the functional and molecular differentiation of synapses and in the mechanisms of plasticity and homeostasis of neuronal circuits. Recently, Mathieu Letellier was awarded a CNRS 2023 bronze medal.
What is your background?
"I have a background in cell biology and physiology through my undergraduate studies at University Pierre and Marie in Paris. Then I did my graduate studies in the laboratory ‘Neurobiology of Adaptive Processes’ with Pr Jean Mariani and Dr Ann Lohof. Finally, I joined Dr Yukiko Goda as a post-doctoral fellow, first at University College London and then at the RIKEN Brain Science Institute in Tokyo. Overall, through my academic career, I have developed a cell physiologist profile [...] and I am now expanding my skills with molecular approaches and high resolution microscopy."
Why did you choose neuroscience?
"Throughout my studies, I was fortunate to have excellent teachers. They passed on me their passion for neuroscience and the will to better understand how the brain works. Moreover, I have chosen neurosciences owing to their interdisciplinary nature. They catalyse strong interactions between people coming from various backgrounds that include cell biology as well as oncology, immunology […] but also chemistry, physics, mathematics, psychology, ethology and many more!"
Why IINS?
"I joined IINS in 2012 following my post-doctorate. At the time, I was looking for a laboratory that would allow me to pursue my research on the development and function of neuronal connections while expanding my field of expertise and knowledge. IINS appeared to be an excellent option: a laboratory in full effervescence, very attractive, open to the world and marked by interdisciplinarity! In addition to that, I had the opportunity to join Olivier Thoumine’s team in which I later obtained a permanent CNRS position. In my opinion, this team perfectly illustrates the spirit of the IINS. Indeed, every members brings a different expertise."
Can you tell us about your research?
"My research has two goals. The first one is to understand how neurons form connections (or synapses) between themselves. The second is to identify the mechanisms by which they modify those connections, either by strengthening or weakening them, to adapt the brain to its environment. These objectives are hampered by the fact that each single neuron harbours a very large number of synapses (10,000 on average) displaying high molecular and functional diversity […]. In the past years, I have been interested in the role of a cell adhesion protein called ‘neuroligin’, whose function is to connect neurons to each other. On the other hand, some mutations in the ‘neuroligin’ genes are associated with autism. In the team, we have shown that the phosphorylation of this protein plays an important role in the differentiation of excitatory synapses but also in long-term synaptic plasticity, the subcellular substrate for learning and memory.”
You have just been awarded the CNRS Bronze Medal 2023. What does this award mean to you?
"To quote the CNRS, this medal ‘rewards the first achievements of researchers who are specialists in their field’. I am beyond honoured to receive this award. In my eyes, it represents: ‘an incentive from the CNRS to continue my research.’ Although this medal is awarded individually, it rewards work in which many people have participated. I thus owe it to my mentors and colleagues I have met and worked with throughout my career, particularly at IINS and within my team."
An advice for young researchers?
"What I wish for the youngest is to find a stimulating and caring laboratory where they can grow professionally and personally. My advice? Find a question that you are passionate about and never lose sight of it. Question yourself, change your point of view, accept failure and contradiction but also trust yourself. Finally, share your research with your colleagues, friends and family: the greatest ideas rarely pop up from a single brain!"
2023-03-23 (MULLE_CARTA Team) : Portrait of Mario Carta, IINS team leader and CNRS researcher

Read the portrait of Mario Carta in English and French
Mario Carta is a native of Sardegna, Italy. He is a neurobiologist with a long-standing interest in synaptic transmission and plasticity in cortical circuits. Recently, he has been focus his research to the study of how cortical circuits encode sensory information. Thus, together with Mikkel Verstergard a postdoc from the laboratory of James Poulet (MDC, Berlin), they published a study in Nature which reports the discovery of a ‘thermal cortex’ located in a posterior region of the insular cortex. This cortical region is involved in the perception of warm and cool.
You recently published an article in Nature. Can you tell us more about it?
"The cortex receive and compute information from the external world to guide our behaviour. Where and how thermal stimuli are processed in the cortex was not known. Together with Mikkel Verstergard, we wanted to understand how non-painful temperatures are encoded at the single cell level in the mammalian cortex. Therefore, we have developed a preparation to optically access the mouse insular cortex. For this purpose, we used large-scale imaging approaches such as wide-field and two-photon calcium imaging in awake behaving mice to record neuronal activity. Finally, in order to confirm the role of the insular cortex in temperature perception, we performed optogenetic manipulations. Thus, our study demonstrated that cooling and warming are coded differently in the mammalian cortex. This highlight the complexity of temperature perception in the brain!"
What is your background?
"I started by studying biology at the University of Cagliari in Italy. Then I obtained the equivalent of a master’s degree in Valenzuela’s laboratory in Albuquerque, USA. Finally, I returned to Italy and obtained a PhD in neuroscience at the
University of Cagliari. [...] Throughout my career, I have a strong background in slice electrophysiology and I have recently shifted my attention to the investigation of neuronal circuits in behaving animals."
Why did you choose neuroscience?
"During my university studies, neuroscience was the subject that interested and stimulated me the most. More specifically, it was by observing a patch clamp electrophysiological recording of a neuron triggering bursts of action potentials that I decided to go into neuroscience."
Why IINS?
"In 2007, I went to Bordeaux for the first time to participate in the "Escube" summer school organised by Christophe Mulle. It was a very enriching experience with quality science and an active scientific community! The following year, I decided to join Christophe’s team as a postdoctoral researcher. Then, in 2013, I obtained a research position at the CNRS. And, from 2017 to 2022, I did a mission and then a secondment in James Poulet’s laboratory. There I studied how in vivo cortical circuits encode sensory information and control behaviour. In 2022, I finally returned to Bordeaux to co-lead the "Synapses and neural circuits" team alongside Christophe. This was a great opportunity for me. Indeed, it allowed me to gain autonomy and to develop my own research. Moreover, this co-direction allowed me to benefit from the collaboration of an experienced and motivated scientist like Christophe."
Tell us about your research
"Currently, I am focusing to studying the cellular mechanisms and circuits underlying taste processing. So, by studying how cortical neurons respond to taste stimuli, I want to understand how the brain processes and integrates sensory information to generate appropriate behavioural responses. This research could have implications for understanding the neural basis of eating behaviour and related disorders."
Any advice for young researchers?
"First of all, choose and develop a research project that you are passionate about and that makes you happy! This will help you stay motivated and dynamic throughout your (long and sometimes difficult) research. Also, find a laboratory with a healthy and positive environment where you can develop personally and professionally."
2023-03-23 (MULLE_CARTA Team) : How does our brain encode cold and warm? by Mario Carta
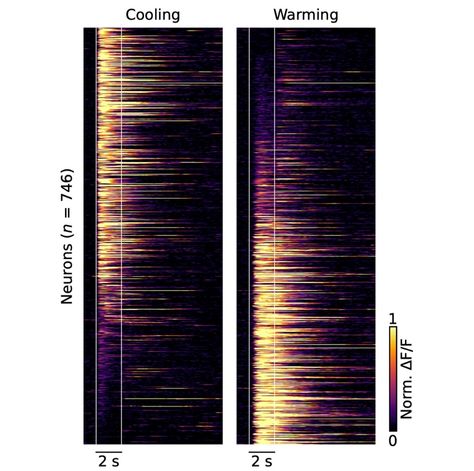 The cellular coding of temperature in the mammalian cortex, Nature - February 2023
The cellular coding of temperature in the mammalian cortex, Nature - February 2023
The cellular coding of temperature in the mammalian cortex.
M. Vestergaard, M. Carta, G. Güney & J. F. A. Poulet - Nature. 2023 Feb 8. doi: 10.1038/s41586-023-05705-5. Online ahead of print.
Warm, cool, these sensations are an integral part of our daily lives. Our ability to detect the temperature of the objects is essential to living. For almost a century, scientists have been trying to determine where in the brain the ability to detect temperatures is located. A study published in Nature reports the discovery of a 'thermal cortex' located in a posterior region of the insular cortex. This cortex can detect cool or warm temperatures.
When our body is in motion, our brain processes the information from our sensory organs. This processing then builds a conscious perception of the world. Much of this process takes place in the outer, folded layer of the brain, called the cortex. The cortex has a major role! It is the house of high brain functions, such as voluntary movement and consciousness.
The scientists started from a previous observation: neurons in the primary somatosensory cortex are active when the skin comes into contact with cool temperatures. They therefore expected that warm temperatures would also be encoded in this region. A test was therefore carried out on mice. To do this, they exposed the front paws of the mice to slight changes in temperature. They then used imaging techniques to find out which part of the brain reacted to these temperature changes. To their surprise, the scientists found that the primary somatosensory cortex did not respond to warming, but on closer inspection, the posterior insular cortex instead responded not only to cooling but also to warming. Using a two-photon microscope, the response of individual neurons in the posterior insular cortex was studied. The scientists discovered that there are cool-specific neurons, warm-specific neurons and neurons that respond to both warm and cool. It should be noted that 'warm' and 'cool' neurons react in very different ways. Indeed, "warm" neurons are sensitive to absolute temperature, while "cool" neurons are activated following a variation in temperature. In addition, the responses of the "cool" neurons are faster than the responses of the "warm" neurons. This indicates that there are potentially separate pathways for the perception of cool and warmth.
To prove conclusively that the insular cortex is involved in temperature perception, the scientists trained mice to respond to cool or warm temperatures. Using optogenetics they were able to temporarily deactivate the posterior insular cortex while delivering a thermal stimulus. In the end, the mice did not feel the thermal stimulus. However, when the scientists stopped deactivating the posterior insular cortex, they felt the stimulus again.
This discovery and the possibility of optically accessing the cortical representation of sensory processing in the insula opens up new avenues of research. First, to study the neural mechanisms of thermal perception, but also to extend similar research to other sensory systems represented in adjacent parts of the insular cortex (in particular the gustatory and visceral systems).
They speak about it too: INSB; CNRS; Bordeaux Neurocampus
Read the portrait of Mario Carta
Contact:
- Mario Carta, chercheur CNRS, mario.carta@u-bordeaux.fr
- James Poulet, professor and group leader, james.poulet@mdc-berlin.de
2023-03-20 (SIBARITA Team) : Job offer: Engineer to develop light-sheet microscopy for neurosciences applications

The Sibarita team "Quantitative Imaging of the Cell" is seeking an engineer to develop light-sheet microscopy for neurosciences applications.
Project description
Light Sheet Fluorescence Microscopy technics (LSFM) have proven to be extremely efficient for 3D imaging of biological samples at various spatial and temporal scales with minimal photo-damaging effects. Several solutions have been developed in the field of neuroscience to image samples ranging from fixed whole brains, to single dissociated neurons growing on a coverslip. In this regard, the Interdisciplinary Institute for Neuroscience (IINS) and the Bordeaux Imaging Center (BIC) are equipped with 3 complementary LSFM techniques: (1) an ultramicroscope for whole brain imging; (2) a Lattice
Light Sheet Microscope (LLSM) to image the first layers of brain slices at high spatial resolution; (3) a home-made single objective selective plane illumination microscope (soSPIM) dedicated to 3D cell cultures and in-depth single-molecule localization microscopy (SMLM).
We aim to complete our catalog by implementing a solution based on the Oblique Plane Microscopy (OPM) architecture, which will be dedicated to fast neuronal samples imaging, ie. brain slices, equipped with local photo-manipulation and possibly other recording modalities.
Missions
The candidate missions will be to:
- develop a custom OPM to address specific neurobiological questions
- participate to the improvement of existing light-sheet microscopes in close collaboration with developers and neuroscientists.
Candidate profile
We seek a motivated, enthusiastic and independent candidate, with an interest in neuroscience and a strong expertise in optics and fluorescence microscopy. Complementary skills in programming and sample preparation are appreciated. The candidate will work in an English-speaking environment, in close interactions with the neuroscientists' team of the Bordeaux's Neurocampus.
Contract
A 3 years research engineer position is available in the framework of the French "Grands Programmes de Recherche" BRAIN awarded to the Bordeaux Neurocampus.
Applicants should send a CV, a motivation letter and contact details for at least two referees to:
jean-baptiste.sibarita@u-bordeaux.fr; remi.galland@u-bordeaux.fr; mathieu.ducros@u-bordeaux.fr
2023-03-03 (SIBARITA Team) : PoCA: a software platform for point cloud data visualization and quantification, Nature - March 2023
Levet, F., Sibarita, JB
Nat Methods. 2023-03-06
https://doi.org/10.1038/s41592-023-01811-4
Point Cloud Analyst (PoCA) is a powerful open-source software platform dedicated to the visualization and analysis of 2D and 3D point-cloud data. PoCA allows manipulating large datasets, and integrates a plugin architecture, a native batch analysis engine and a Python code interpreter, facilitating both the analysis of data and the integration of new methods.
PoCA is packaged as a one-click installer for the Windows operating system. The source code is available on GitHub (https://github.com/flevet/PoCA) under a LGPL v3 license. We provide a cmake script to facilitate its building inside other operating systems. The documentation is available at https://poca-smlm.github.io/.
To know more:
2023-02-24 : Call for applications: Master EUR Light Sciences and Technology, Biophotonics and Neurotechnology
The call is now open and candidates should send their applications to master.applications.light-st@u-bordeaux.fr
The master track “Biophotonics and Neurotechnology” is part of the Light Sciences and Technologies Graduate Program at the University of Bordeaux (EUR or Ecole Universitaire de Recherche and the ‘Mention: Ingénierie de la santé’). This one offers a multidisciplinary training program on advanced microscopy and photonics tools to address complex biological problems in the life sciences, in particular neuroscience and cancer biology.
For who ? Biology, physics, chemistry, engineering undergraduate students, bio-techno-philes of all stripes.
The academic program is composed of:
- basic and specialised lecture and practicum courses,
- internships in active research laboratories and core facilities equipped with state-of-the-arts technology and microscopes,
- supporting infrastructure needed for modern research.
The courses and practicals are run by leading research faculty and staff, who take pride and interest in teaching and forming the next generation of biophotonics researchers and engineers. The program is run in English and the students study and work in the international, collaborative and diverse research community of Bordeaux Neurocampus.
Key points
- Multidisciplinary training in biophotonics, its technology and application
- An identified area of research excellence at the University of Bordeaux
- Taught by strong research faculty (including several ERC laureates)
- Financial support offered, M1 and M2 internships are paid + possibility for merit-based fellowships (750€ / month)
- Career perspectives in academic research (e.g. doctoral school) and private industry, as technology and application specialist, in R&D, sales and service sectors
Moreover, the program offers financial support to all enrolled students (in the form of merit-based “excellence fellowships” and paid internships) and a variety of career opportunities (workshops, summer schools and research stays in France and abroad).
For candidate: master.applications.light-st@u-bordeaux.fr
More information
- https://formations.u-bordeaux.fr/#/details-formation?type=parcours-type&id=1635
- https://light-st.u-bordeaux.fr/
- Light Sciences and Technologies Graduate Program
Contact: Valentin Nägerl, team leader IINS and University professor - Researcher at the Université de Bordeaux
2023-02-20 (SIBARITA Team) : A framework for evaluating the performance of SMLM cluster analysis algorithms - Nature, Feb 2023

Daniel J. Nieves, Jeremy A. Pike, Florian Levet, David J. Williamson, Mohammed Baragilly, Sandra Oloketuyi, Ario de Marco, Juliette Griffie, Daniel Sage, Edward A. K. Cohen, Jean-Baptiste Sibarita, Mike Heilemann, Dylan M. Owen
Nat Methods. 2023-02-01; 20(2): 259-267
DOI: 10.1038/s41592-022-01750-6
Single-molecule localization microscopy (SMLM) generates data in the form of coordinates of localized fluorophores. Cluster analysis is an attractive route for extracting biologically meaningful information from such data and has been widely applied. Despite a range of cluster analysis algorithms, there exists no consensus framework for the evaluation of their performance. Here, we use a systematic approach based on two metrics to score the success of clustering algorithms in simulated conditions mimicking experimental data. We demonstrate the framework using seven diverse analysis algorithms: DBSCAN, ToMATo, KDE, FOCAL, CAML, ClusterViSu and SR-Tesseler. Given that the best performer depended on the underlying distribution of localizations, we demonstrate an analysis pipeline based on statistical similarity measures that enables the selection of the most appropriate algorithm, and the optimized analysis parameters for real SMLM data. We propose that these standard simulated conditions, metrics and analysis pipeline become the basis for future analysis algorithm development and evaluation.
The point of view of Jean-Baptiste Sibarita and Florian Levet (co-authors)
This work by Nieves et al. proposes metrics to evaluate the performances of clustering methods dedicated to single molecule localization microscopy (SMLM) data. It includes the methods and several reference simulation datasets to assess the performances of existing and future approaches. This is a consortium paper that regroups word wide recognized experts in the field of SMLM. Amongst them, Florian Levet and Jean-Baptiste Sibarita from the Quantitative imaging of the Cell team (IINS) & Bordeaux Imaging Center, contributed to this work as developers of SR-Tesseler and Coloc-Tesseler software and the corresponding methods published in (Levet et al, Nature Methods 2015) and (Levet et al, Nature Comm, 2019).
2023-02-17 : The 2022 highlights by la Societe des Neurosciences and IINS

La Societe des Neurosciences is a non-profit scientific association, governed by the law of 1901. Its aim is to promote the development of research in all areas of neuroscience. The association brings together scientists and physicians from all over the world whose research and work are focused on the study of the brain and the nervous system.
Every year, la Societe des Neurosciences selects the most outstanding publications in the field of neuroscience. All members of la Societe des Neurosciences are invited to submit articles they have written or read. In 2022, among the 20 selected highlights, there are two highlights where IINS is mentioned.
1. Les synapses pivot a dopamine dans le striatum : un nouveau point nevralgique pour la neuromodulation par la dopamine ?
Vincent Paget-Blanc, Marlene E Pfeffer, Marie Pronot, Paul Lapios, Maria-Florencia Angelo, Roman Walle, Fabrice P Cordelieres, Florian Levet, Stéphane Claverol, Sabrina Lacomme, Melina Petrel, Christelle Martin, Vincent Pitard, Veronique De Smedt Peyrusse, Thomas Biederer, David Perrais, Pierre Trifilieff, Etienne Herzog
Nature Communications. 2022 Jun 3;13(1):3102. 10.1038/s41467-022-30776-9.
2. Une nouvelle boite a outils pour explorer la dynamique des recepteurs dans le cerveau
Angela Getz, Mathieu Ducros, Christel Breillat, Aurelie Lampin-Saint-Amaux, Sophie Daburon, Urielle François, Agata Nowacka, Monica Fernandez Monreal, Eric Hosy, Frederic Lanore, Hanna Zieger, Mathieu Sainlos, Yann Humeau, Daniel Choquet
Science Advances. 2022 Jul 29; 8(30):eabm5298. 10.1126/sciadv.abm5298
Learn more about our research team
2023-02-12 (ELEGHEERT Team) : Jonathan Elegheert is 2022 Laureate FSER
 Two laureates of the FSER at IINS: Lisa Roux and Jonathan Elegheert
Two laureates of the FSER at IINS: Lisa Roux and Jonathan Elegheert
Created on the initiative of a group of scientists wishing to have fundamental research recognised, the Cercle FSER is an association under the French law of 1901. It brings together more than 70 scientists who are committed to fostering the dialogue of science with and for society. To this end, its actions are organised along two main lines: (1) to promote research, its approaches and its challenges to young people, (2) to encourage the involvement of research staff in dialogue with the general public. In 2021, 35,000 people benefited from its actions!
Each year, the Cercle FSER supports young researchers who excel in the field of biomedical research. The candidates are nominated by scientific personalities, former laureates or the CNRS and INSERM. Then, each candidate is selected by a jury on the basis of the quality of his or her past and present scientific research, but also on the basis of his or her performance in the interview.
Thus, Jonathan Elegheert, CNRS scientist (charge de recherches), is one of four laureates of 2022 Cercle FSER! In particular, he obtained the FSER prize, which aims to help young researchers during the first years of their laboratory's creation. This grant enabled Jonathan Elegheert and his team to co-finance a new machine to study protein-protein interactions.
In 2021, Lisa Roux, CNRS research director, was laureate. Like any laureate, the Cercle FSER has enabled her to organise meetings at the Fondation des Treilles! Moreover, as a winner of the Cercle FSER, her objective for 2023 is to participate in the Déclics operation (scientific mediation action with high school students).
One per year, the Cercle FSER organises meetings so that researchers from all disciplines can meet. These exchanges allow them to come up with new and original ideas! The winter meeting took place from 24 to 27 January in Alpe d'Huez. During this meeting, all participants were invited to make a scientific presentation. Thus, Jonathan Elegheert and Lisa Roux intoduced the field of neuroscience! Both presented their research topic: "Structural biology and engineering of neuronal proteins" for Jonathan Elegheert and "Olfaction and Memory" for Lisa Roux.
Would you like to know more? Nolwenn Cloarec, communication officer: nolwenn.cloarec@u-bordeaux.fr
2023-02-12 (NAGERL Team) : Imaging dendritic spines in the hippocampus of a living mouse by 3D-STED microscopy - February 2023
2023-02-07 (MULLE_CARTA Team) : Christophe Mulle receives the Prix Desmarest

Le Prix Desmarest of the Pierre Deniker Foundation, aims to fund basic research projects in the field of Alzheimer's disease and neurodegenerative diseases, with a total endowment of €100,000. The Pierre Deniker Foundation supports mental health research programs, and raises public awareness of mental disorders.
For this third edition, the Prix Desmarest was awarded to Christophe Mulle, CNRS research director. The project, which will be co-headed by Thierry Amedee, aims to investigate the morpho-functional relationships between microglia and synapses near amyloid plaques in an animal model of Alzheimer's disease. Christophe Mulle was awarded the prize on January 20th at the Encephale Congress (Paris) by Annick Desmarest and by Chantal Henry, scientific director of the Pierre Deniker Foundation.
Learn more about: Christophe Mulle and Thierry Amedee
Do you want to know more? Nolwenn Cloarec, communication officer: nolwenn.cloarec@u-bordeaux.fr

2023-02-02 (CHOQUET Team) : Daniel Choquet: prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux

Academie nationale des sciences, belles lettres et arts de Bordeaux aims to help develop the ideas, work and research of Academicians. Each year, it rewards in particular personalities for their work or their research or for all of their work in the field of science, literature or the arts.
Thus, in 2022, it was Daniel Choquet, CNRS research director, who was awarded "le grand prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux"! Indeed, he achieved this distinction thanks to his main scientific achievement: the discovery that neurotransmitter receptors are in constant motion in the neuronal membrane and that the regulation of this traffic profoundly regulates synaptic transmission. Daniel Choquet will receive his award on March 28 from Pierre Hurmic, Mayor of Bordeaux.
Learn more about: Le grand prix 2022 de l’Academie nationale des sciences, belles lettres et arts de Bordeaux
They talk about it too (articles in French): INSB and Bordeaux Neurocampus
Do you want to know more? Nolwenn Cloarec, communication officer: nolwenn.cloarec@u-bordeaux.fr
2022-06-08 : TEST SEND TITLE BY MAIL
None
2022-05-19 (MULLE_CARTA Team) : Corlieve Therapeutics, co-funded by Christophe Mulle acquired by the biotech uniQure:
 uniQure to Acquire Corlieve Therapeutics
uniQure to Acquire Corlieve Therapeutics
Corlieve Therapeutics co-funded by Christophe Mulle and Valérie Crépel (Inserm Marseille) acquired by the biotech uniQure. The acquisition of Corlieve Therapeutics by uniQure amounts to 250 million euros, of which the first initial settlement of 46.3 million euros finalizes the transaction.
https://www.bordeaux-neurocampus.fr/en/uniqure-to-acquire-corlieve-therapeutics/
2022-03-03 (MULLE_CARTA Team) : Awarded 2022 Prize for Innovation of the Académie des Sciences et Belles Lettres de Bordeaux


Valérie Crépel (INSERM) at the University of Aix-Marseille and Christophe Mulle (CNRS) at the Interdisciplinary Institute for Neuroscience at the University of Bordeaux have been awarded the 2022 Prize for Innovation of the Académie des Sciences et Belles Lettres de Bordeaux.
Valérie Crépel and Christophe Mulle are very high-level researchers interested in the physiology and pathology of the hippocampus circuits, a region of the brain located in the temporal lobes, which is the seat of memory and learning.
Their highly innovative research focuses on the therapeutic approach to the treatment of temporal lobe epilepsy. This pathology affects 1.3 million people in Europe and the United States, including 800,000 patients who are resistant to known treatments. Christophe Mulle and Valérie Crépel propose gene therapy as an alternative to surgery and its drawbacks. Their therapeutic proposal is developed within Corlieve Therapeutics: it uses microRNA technology, nucleic acids capable of acting at the level of messenger RNA to selectively reduce aberrant effects in the hippocampus of patients.
2021-09-13 (PERRAIS Team) : Post-synaptic exocytosis and synaptic plasticity - Cell Reports Sept 2021

Synapses, the basic building blocks of neural networks, are both very stable and capable of rapid and long-lasting modifications, a phenomenon known as synaptic plasticity. The modification of a synapse often involves the addition of synaptic receptors (long-term potentiation or LTP) or the removal of part of the synaptic receptors (long-term depression or LTD). This rapid plasticity is possible because synaptic receptors are not immobile in the synapse but travel to intracellular compartments called recycling endosomes (RE). The regulation of RE trafficking has thus become an important topic of study for understanding the mechanisms of synaptic plasticity.

Picture of all contributors of this study. David Perrais and May Bakr stand in front of "The paint pipette", taken by May, which was awarded the best IINS picture prize in 2020.
In this study, we searched for the molecules involved in these phenomena, in particular the proteins responsible for exocytosis called SNAREs. The VAMP2 protein, target of the tetanus toxin (released by the bacterium responsible for tetanus and one of the most deadly in humans), was known to block LTP. However, to our surprise, it only marginally affected RE exocytosis. We therefore searched for other SNARE proteins and found VAMP4 to be responsible for the majority of RE exocytosis, whereas VAMP2 is involved in only a small fraction of exocytosis, but plays a major role in the exocytosis of REs containing AMPA-type postsynaptic receptors (see Figure). Furthermore, VAMP4 deletion also alters the trafficking of AMPA receptors that are in greater quantity at the surface of neurons, increasing synaptic transmission and limiting its plasticity by occlusion.
This work shows the great diversity of membrane trafficking mechanisms in the dendrites of neurons that allows receptors to be delivered when and where they are needed to regulate individual synapses. It was the result of a long-term work, over more than eight years, of many students, engineers and researchers of the IINS.
2021-09-07 (MULLE_CARTA Team) : Celebrating the sale of Corlieve Therapeutics
 The startup Corlieve Therapeutics co-founded by Christophe Mulle PI @IINS just sold to UNIQURE for 250 M€
The startup Corlieve Therapeutics co-founded by Christophe Mulle PI @IINS just sold to UNIQURE for 250 M€
Happy to celebrate the sale of Corlieve Therapeutics for 250 M€, the startup created by Christophe Mulle and Valérie Crépel from their work partly at IINS.
Corlieve Therapeutics is a biotechnology company focused on bringing novel therapeutic options to patients with severe neurological disorders. The lead project is targeting aberrantly expressed kainate receptors in the hippocampus of patients with refractory Temporal Lobe Epilepsy using a gene therapy approach.
More info here
2021-09-03 (ROUX Team) : Lisa gave a talk at the 3rd Science and Gender Equality Symposium “SAGE 3.0”
Dates: October 21-22, 2021 on ZOOM. The symposium is funded by the DFG Collaborative Research Center 936 (SFB 936). To know more, follow the link below.
2021-08-30 (ROUX Team) : Lisa Roux laureate of the Fondation Schlumberger pour l'Education et la Recherche

The Schlumberger Foundation for Education and Research selects each year three laureates amongst the best young researchers who are building their team in the field of life sciences. A group leader of IINS, Lisa Roux, is one of the laureates in 2021. She has just received a prize to support the establishment of her team and will join the Cercle FSER in taking action to defend and highlight the value of basic science research.
2021-08-30 (ROUX Team) : Evan Harrell is joining the team with an IdEx Junior Chair of Bordeaux University
Congratulations to him!
2021-05-25 (GIANNONE Team) : Orré T., Rossier O. and Giannone G. in Nature Communications - May 2021
 Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions
Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions
Focal adhesions (FAs) initiate chemical and mechanical signals involved in cell polarity, migration, proliferation and differentiation. Super-resolution microscopy revealed that FAs are organized at the nanoscale into functional layers from the lower plasma membrane to the upper actin cytoskeleton. Yet, how FAs proteins are guided into specific nano-layers to promote interaction with given targets is unknown. Using single protein tracking, super-resolution microscopy and functional assays, we link the molecular behavior and 3D nanoscale localization of kindlin with its function in integrin activation inside FAs. We show that immobilization of integrins in FAs depends on interaction with kindlin. Unlike talin, kindlin displays free diffusion along the plasma membrane outside and inside FAs. We demonstrate that the kindlin Pleckstrin Homology domain promotes membrane diffusion and localization to the membrane-proximal integrin nano-layer, necessary for kindlin enrichment and function in FAs. Using kindlin-deficient cells, we show that kindlin membrane localization and diffusion are crucial for integrin activation, cell spreading and FAs formation. Thus, kindlin uses a different route than talin to reach and activate integrins, providing a possible molecular basis for their complementarity during integrin activation.
 Contacts IINS: Olivier Rossier and Grégory Giannone
Contacts IINS: Olivier Rossier and Grégory Giannone
2021-03-16 (ROUX Team) : Lisa interviewed for article in Horizon Magazine
The EU Research and Innovation Magazine
On the role of social scents. Article by Alex Whiting.
2021-03-16 (ROUX Team) : Lisa and Pascal contributed to "L'Enquête des Sens"
Interviews (in French) on olfactory perception and the link between olfaction and memory.
Chaque année, les étudiant·es du master Médiation des sciences de Bordeaux et l’association Dealers de Sciences organisent une semaine de culture scientifique et technique. Pour sa 5ème édition, la thématique choisie est « L’Enquête des Sens ». Lisa Roux et Pascal Ravassard ont répondu aux questions de leur interviewers sur la perception olfactive (https://www.lenquetedessens.dealersdescience.com/index.php/2020/10/03/les-serrures-de-lodorat/) et le lien entre mémoire et odorat (https://www.lenquetedessens.dealersdescience.com/index.php/2020/11/08/les-echos-de-la-memoire/).
2021-03-14 (CHOQUET Team) : Nature Neuroscience Review - Choquet D., Sainlos M. and Sibarita J.B.
 Nature Neuroscience Review - Advanced imaging and labelling methods to decipher brain cell organization and function
Nature Neuroscience Review - Advanced imaging and labelling methods to decipher brain cell organization and function
We review the latest developments for labelling and functionalizing proteins with small localization and functionalized reporters. We present how these molecular tools are combined with the development of a wide variety of imaging methods that break either the diffraction barrier or the tissue penetration depth limits. We put these developments in perspective to emphasize how they will enable step changes in our understanding of the brain.
2021-01-14 (PERRAIS Team) : MemTraS Team crew is expanding
New members join the team for 2021
We are pleased to welcome several new members:
Sarka Jelinkova - Post Doctoral Fellow - Expert in Stem cell biology and molecular biology - Ph.D U Brno - Czech Republic
Nathan Hoareau - Master 2 rotation student - Ecole Supérieure de Chimie Organique et Minerale
Juan Manuel Defauce Garcia - Master 1 NeuroBIM rotation student - Université de Bordeaux
Khadija Inam - Master 1 NeuroBIM rotation student - Université de Bordeaux
Fantine Morinière - Technician degree rotation student - BTS Biotechnologies - Lycée Saint-Louis - Bordeaux
Welcome to all of them!
2021-01-13 (MULLE_CARTA Team) : The relationship between brain state and membrane potential in CA3 pyramidal cells
Malezieux, Kees, and Mulle, Cell Reports 2020
Wakefulness is comprised of distinct brain states correlated with different behaviors and stages of memory. It is hypothesized that memory encoding and recall are more prominent during active behaviors, while memory consolidation is more prominent during rest. The hippocampal region of the brain is involved in all these stages of memory during their respective brain states. For this brain circuit to perform these different computations at different times, it has been hypothesized that the membrane potential of individual neurons must change in a brain state-dependent manner. We sought to test this hypothesis by recording membrane potential from individual CA3 hippocampal pyramidal cells in awake mice during active and restful behaviors. When animals are actively moving, the hippocampal local field potential displays a 4-12 Hz oscillation known as theta. We found that, consistent with the hypothesis, CA3 pyramidal cells underwent consistent changes in membrane potential when theta was present in the local field potential. Specifically, these cells hyperpolarized, decreased firing, and had low membrane potential variance, all of which are consistent with increased inhibition. This sustained, coherent suppression of CA3 pyramidal cells during theta likely changes the circuit dynamics within the hippocampus, contributing to a functional switch that might underlie the ability of the hippocampus to participate in memory encoding during theta.
- Cell Reports 2020 - doi: 10.1016/j.celrep.2020.107868
- Authors and contacts: Meryl Malezieux, Ashley L Kees and Christophe Mulle
+ Cf Bordeaux Neurocampus website here
2021-01-05 (NAGERL Team) : Award of ERC Synergy Grant
Together with my Bordeaux colleagues (E. Bezard, L. Cognet and L. Groc), I am a proud recipient of an ERC Synergy grant from the EU, which supports high-risk/high gain frontier research in Europe. This is a tremendous recognition of our work, which will give us resources and wings to conduct some cool and ground-breaking research in the years to come! I am extremely grateful to all the members of my team and collaborators who have contributed to this success over the years and made it possible.
2020-09-26 (CHOQUET Team) : Multicolor Spectrin labeling (ML Jobin)
This is a rat hippocampal neuron in culture stained with a series of markers

2020-09-22 : Synaptic plasticity and the recovery of altered skills
 Cell Reports, Sept. 2020
Cell Reports, Sept. 2020
AMPAR-Dependent Synaptic Plasticity Initiates Cortical Remapping and Adaptive Behaviors during Sensory Experience
Cortical plasticity improves behaviors and helps recover lost functions after injury. However, the underlying synaptic mechanisms remain unclear. In mice, we show that trimming all but one whisker enhances sensory responses from the spared whisker in the barrel cortex and occludes whisker-mediated synaptic potentiation (w-Pot) in vivo. In addition, whisker-dependent behaviors that are initially impaired by single-whisker experience (SWE) rapidly recover when associated cortical regions remap. Cross-linking the surface GluA2 subunit of AMPA receptors (AMPARs) suppresses the expression of w-Pot, presumably by blocking AMPAR surface diffusion, in mice with all whiskers intact, indicating that synaptic potentiation in vivo requires AMPAR trafficking. We use this approach to demonstrate that w-Pot is required for SWE-mediated strengthening of synaptic inputs and initiates the recovery of previously learned skills during the early phases of SWE. Taken together, our data reveal that w-Pot mediates cortical remapping and behavioral improvement upon partial sensory deafferentation.
Authors: Tiago Campelo, Elisabete Augusto, Nicolas Chenouard, Come Camus, Daniel Choquet, Frédéric Gambino
- Cell Reports, Sept. 2020 - DOI:https://doi.org/10.1016/j.celrep.2020.108097
- Contacts: Frédéric Gambino and Daniel Choquet
2020-09-01 (ELEGHEERT Team) : Synthetic excitatory synaptic organizer - Science, August 2020
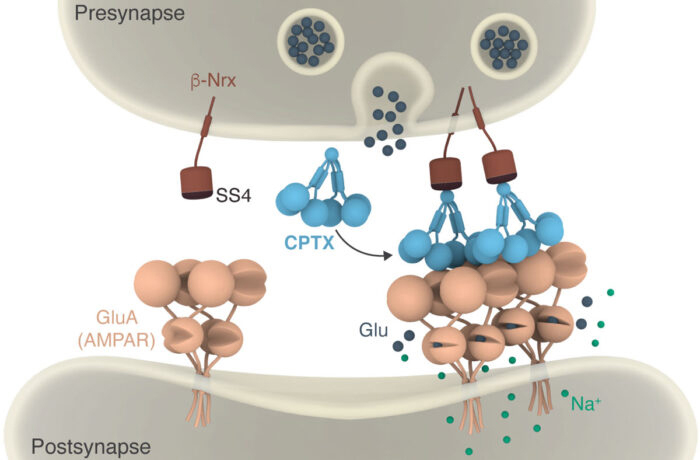 Science, August 2020
Science, August 2020
A synthetic synaptic organizer protein restores glutamatergic neuronal circuits
The human brain contains trillions of synapses within a vast network of neurons. Synapse remodeling is essential to ensure the efficient reception and integration of external stimuli and to store and retrieve information. Building and remodeling of synapses occurs throughout life under the control of synaptic organizer proteins. Errors in this process can lead to neuropsychiatric or neurological disorders. Suzuki et al. combined structural elements of natural synaptic organizers to develop an artificial version called CPTX, which has different binding properties (see the Perspective by Salinas). CPTX could act as a molecular bridge to reconnect neurons and restore excitatory synaptic function in animal models of cerebellar ataxia, familial Alzheimer’s disease, and spinal cord injury. The findings illustrate how structure-guided approaches can help to repair neuronal circuits.
Authors: Kunimichi Suzuki, Jonathan Elegheert, Inseon Song, Hiroyuki Sasakura, Oleg Senkov, Keiko Matsuda, Wataru Kakegawa, Amber J. Clayton, Veronica T. Chang, Maura Ferrer-Ferrer, Eriko Miura, Rahul Kaushik, Masashi Ikeno, Yuki Morioka, Yuka Takeuchi, Tatsuya Shimada, Shintaro Otsuka, Stoyan Stoyanov, Masahiko Watanabe, Kosei Takeuchi, Alexander Dityatev, A. Radu Aricescu, Michisuke Yuzaki
- Science, 28 Aug 2020: Vol. 369, Issue 6507, eabb4853 - DOI: 10.1126/science.abb4853
- Contact: Jonathan Elegheert
2020-07-20 : A new website for the Aquitaine Delegation of the CNRS
On the new Aquitaine Delegation website find dedicated pages to the organization of the Delegation, scientific potential, innovation, as well as an agenda and a News section here
2020-07-01 (GROC Team) : Retrovirus, inflammation and psychosis: a missing link identified!
 Science Advances, July 2020
Science Advances, July 2020
Human endogenous retroviral protein triggers deficit in glutamate synapse maturation and behaviors associated with psychosis
Mobile genetic elements, such as human endogenous retroviruses (HERVs), produce proteins that regulate brain cell functions and synaptic transmission and have been implicated in the etiology of neurological and neurodevelopmental psychiatric disorders. However, the mechanisms by which these proteins of retroviral origin alter brain cell communication remain poorly understood. Here, we combined single-molecule tracking, calcium imaging, and behavioral approaches to demonstrate that the envelope protein (Env) of HERV type W, which is normally silenced but expressed in patients with neuropsychiatric conditions, alters the N-methyl-d-aspartate receptor (NMDAR)–mediated synaptic organization and plasticity through glia- and cytokine-dependent changes. Env expression in the developing hippocampus was sufficient to induce behavioral impairments at the adult stage that were prevented by Env neutralization or tuning of NMDAR trafficking. Thus, we show that a HERV gene product alters glutamate synapse maturation and generates behavioral deficits, further supporting the possible etiological interplay between genetic, immune, and synaptic factors in psychosis.
Authors: E.M. Johansson, D. Bouchet, R. Tamouza, P. Ellul, AS. Morr, E. Avignone, R. Germi, M. Leboyer, H. Perron and L. Groc
- Science Advances 17 Jul 2020: Vol. 6, no. 29, eabc0708 - DOI: 10.1126/sciadv.abc0708
- Contacts: Emily M. Johannson and Laurent Groc
+ More details on the Neurocampus website here
2020-04-07 : Information : Coronavirus
The French Academy of Sciences provides links to find reliable information on the COVID-19 Coronavirus epidemic.
2020-03-16 : IINS is temporarily closed

Our lab has closed down to allow the personnel to go under confinement and protection. A handful of dedicated members is taking care of our precious biological samples and mouse colonies.

